FDA launches safety review of RSV infant drugs as RFK Jr. scrutinizes immunizations
NeutralHealth
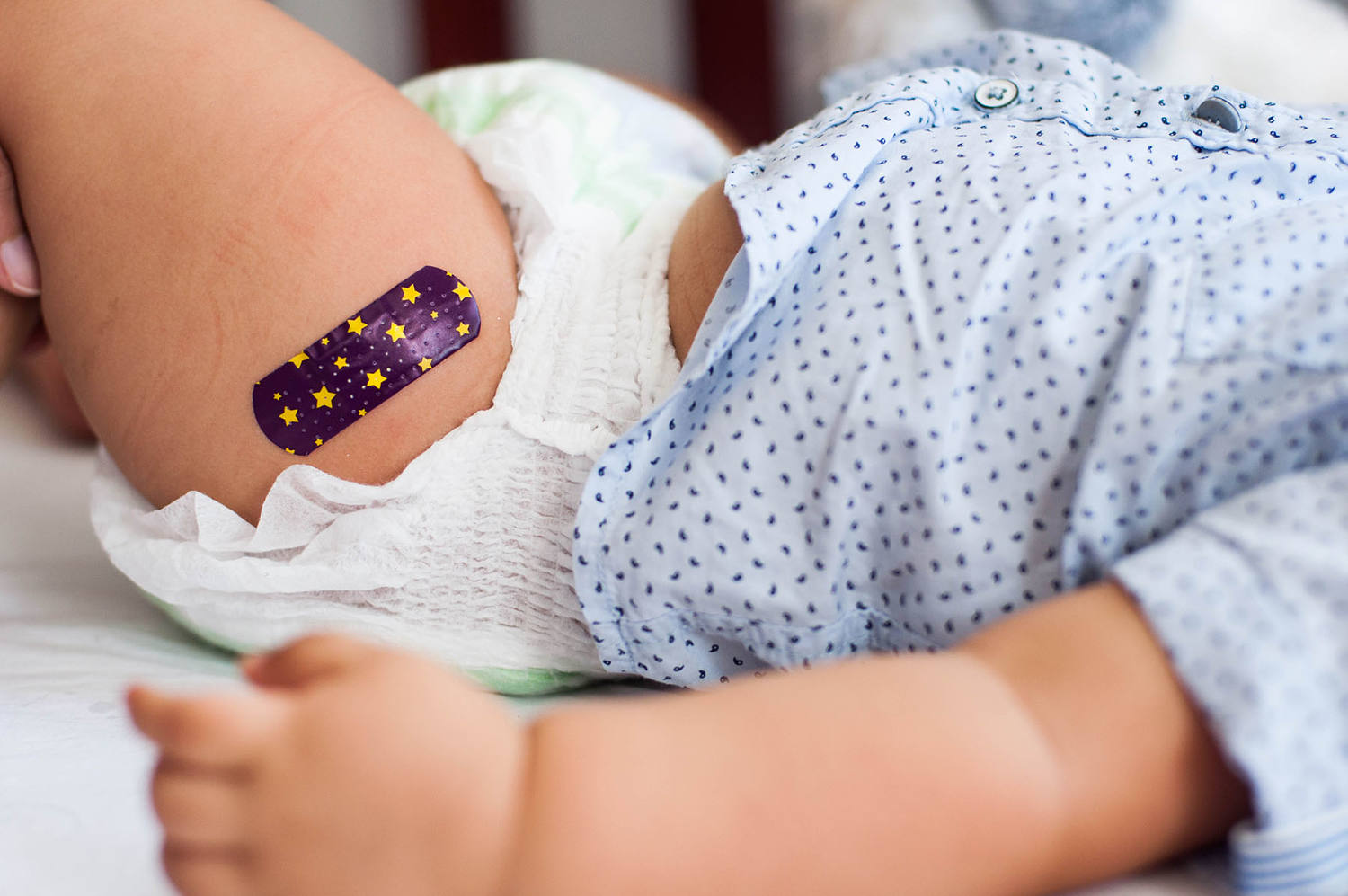
- The Food and Drug Administration (FDA) has initiated a safety review of two approved drugs for respiratory syncytial virus (RSV) in infants, coinciding with increased scrutiny of immunizations led by Health Secretary RFK Jr.
- This review is significant as it reflects ongoing concerns regarding the safety and efficacy of infant immunizations, particularly in light of RFK Jr.'s controversial stance on vaccines, which has drawn both support and criticism from various health advocates.
- The scrutiny surrounding these immunizations is part of a broader debate on vaccine safety, highlighted by recent discussions on delaying the hepatitis B vaccination for newborns and the backlash against misinformation at health advisory meetings, indicating a growing tension in public health policy.
— via World Pulse Now AI Editorial System
