FDA approves label change for Depo-Provera, adding brain tumor warning
NegativeHealth
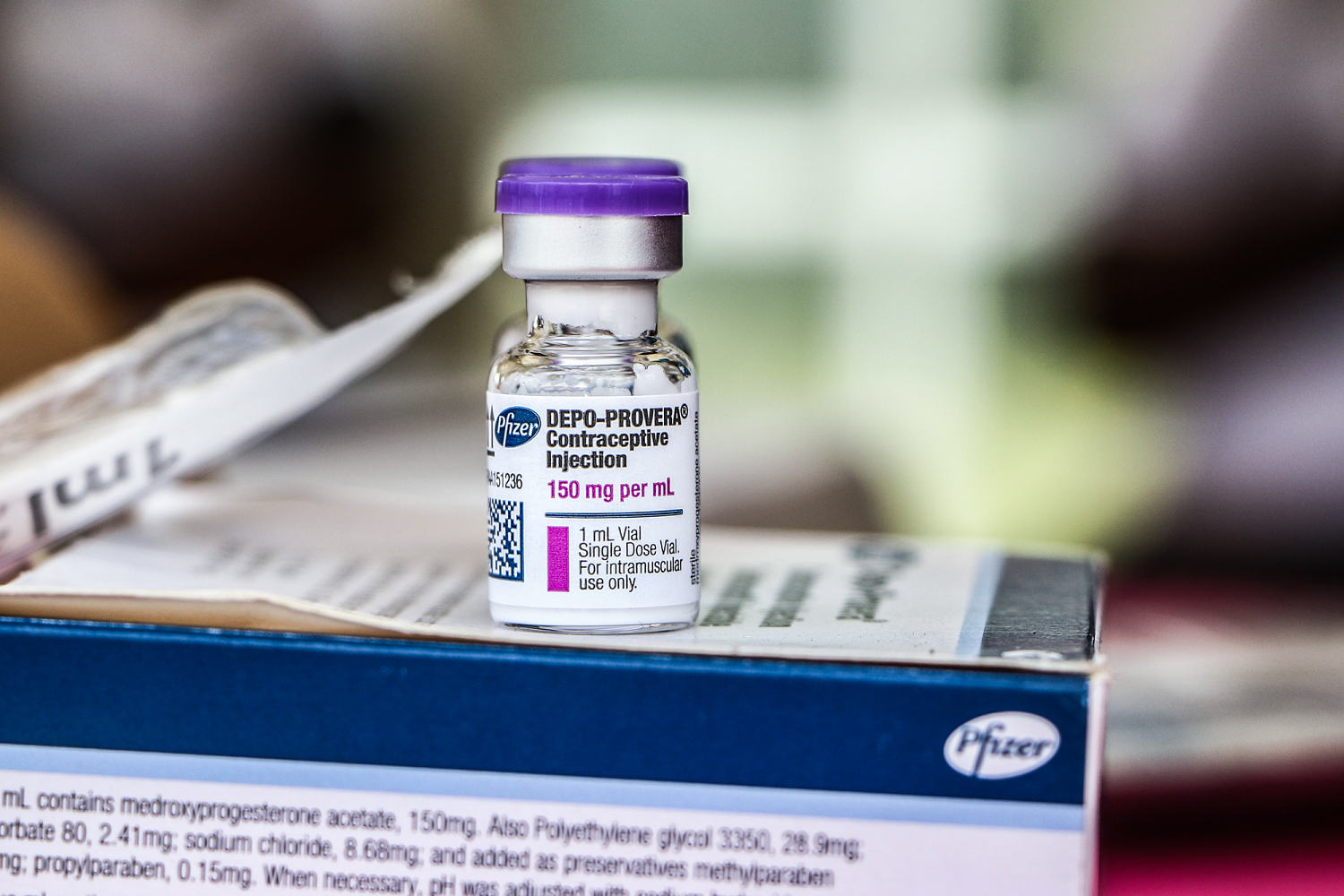
- The FDA has approved a label change for Pfizer's birth control shot, Depo-Provera, to include a warning about the risk of meningioma, a type of brain tumor. This decision follows growing concerns regarding the safety of the contraceptive and its potential health implications for users.
- This label change is significant for Pfizer as it may impact the perception and sales of Depo-Provera, which has been a widely used contraceptive. The warning could lead to increased scrutiny and legal challenges, as patients may seek more information about the risks associated with the drug.
- The development highlights ongoing debates about pharmaceutical transparency and the responsibilities of drug manufacturers to inform patients about potential risks. The emergence of lawsuits alleging that Pfizer failed to adequately warn women about the tumor risk underscores the critical need for clear communication regarding medication safety.
— via World Pulse Now AI Editorial System

